Imaging Pearls ❯ Deep Learning ❯ Machine Learning
|
-- OR -- |
|
- “ Rajpurkar emphasizes the importance of understanding the “data generation process,” including the artifacts and biases baked into data, which is illustrated by a specific example where an AI model exploited metadata rather than clinically relevant features. Dr. Rajpurkar addresses the urgent need for more open and accessible medical data with his initiative on Medical AI Data for All (MAIDA). We also examine the changing role of clinicians in an AI-augmented health care system, and discuss a collaborative approach where human expertise guides AI development and implementation. Looking ahead, we envision a future where AI systems generate comprehensive medical reports and engage in natural language interactions, while emphasizing the need for ongoing focus on safety, efficacy, and equitable access. ”
Pixels and Pitfalls: Building Robust Artificial Intelligence for Medical Imaging
Pranav Rajpurkar , Andrew L. Beam , Arjun K. Manrai
NEJM AI 2024; 1 (10) - “Collaboration between clinicians and AI researchers remains crucial. Clinicians play a key role in problem definition, ensuring that AI systems address real clinical needs and account for the full complexity of medical tasks. Interdisciplinary teams of young researchers and senior clinical collaborators have had substantial impact.”
Pixels and Pitfalls: Building Robust Artificial Intelligence for Medical Imaging
Pranav Rajpurkar , Andrew L. Beam , Arjun K. Manrai
NEJM AI 2024; 1 (10) - “The siloed nature of medical data and privacy concerns are major obstacles, potentially holding us back by a decade or more. We urgently need a paradigm shift toward open, accessible medical data for research, balancing privacy concerns with the immense potential for improving health care through AI. Initiatives such as MAIDA aim to create a global, standardized dataset for evaluating AI models with the goal of accelerating model development, similar to that which has been done in the general machine learning community.”
Pixels and Pitfalls: Building Robust Artificial Intelligence for Medical Imaging
Pranav Rajpurkar , Andrew L. Beam , Arjun K. Manrai
NEJM AI 2024; 1 (10) - “As AI capabilities grow, the role of clinicians will inevitably evolve. Rather than fearing replacement, health care professionals should embrace AI as a powerful tool, focusing on problem definition and the nuanced aspects of patient care that machines cannot replicate. The future likely lies in seamless human–AI collaboration, with clinicians guiding the development, implementation, and use of these technologies.”
Pixels and Pitfalls: Building Robust Artificial Intelligence for Medical Imaging
Pranav Rajpurkar , Andrew L. Beam , Arjun K. Manrai
NEJM AI 2024; 1 (10) - “ While artificial general intelligence (AGI) and artificial superintelligence (ASI) remain speculative, the possibility of capabilities that meet or surpass expert levels in areas such as treatment planning, clinical reasoning, and cognitive empathy merits consideration and debate in anticipation of the day when these capabilities become viable. Current ethical debates about AI often center on immediate concerns like data bias, but the progression toward AGI and ASI presents a profound and novel challenge: these systems might develop ethical frameworks that fundamentally differ from human-derived ethics. Planning for them must anticipate these changes, ensuring that their ethical paradigms uphold human values. Given the transformative potential of AGI and ASI, a multidisciplinary dialogue among medical professionals, policy makers, and technology experts is essential to prepare for these advancements.”
If Machines Exceed Us: Health Care at an Inflection Point
Eyal Klang
NEJM AI 2024; 1 (10
- “Weakly supervised learning reduces human supervision in the development of DL models. However, this may make the generalizability of model performance and the certainty of the model outputs more opaque compared with supervised learning approaches. Consequently, it is crucial to validate weak supervision–trained models with accurate reference standard labels and to investigate what level of human supervision is necessary in the clinical use of these models.”
Weakly Supervised Deep Learning in Radiology
Leo Misera • Gustav Müller-Franzes• Daniel Truhn• Jakob Nikolas Kather
Radiology 2024; 312(1):e232085 - “The bottleneck for using deep learning (DL) models in radiology is often not the acquisition of a large number of images but rather obtaining the labels. Weakly supervised DL marks a shift from using manually obtained complete, exact, and accurate labels to leveraging data sets with incomplete, inexact, or inaccurate labels that require little or no additional manual annotation from experts. With this shift, it becomes feasible to leverage vast clinical databases to swiftly construct large and balanced data sets for model training. With careful validation of the trained models, weakly supervised learning could accelerate the development of DL models for clinical applications including diagnosis, prognosis, and segmentation.”
Weakly Supervised Deep Learning in Radiology
Leo Misera • Gustav Müller-Franzes• Daniel Truhn• Jakob Nikolas Kather
Radiology 2024; 312(1):e232085 - Essentials
■ Weakly supervised learning enables scalability in radiology by using incomplete, inexact, or inaccurate labels, thus reducing or entirely removing the need for manual labeling.
■ Vast data sets can be obtained automatically by using labels extracted from free-text radiology or pathology reports; these labels may be incomplete, inexact, or inaccurate.
■ Weakly supervised learning accelerates the development of deep learning models for tasks such as diagnosis, prognostication, and segmentation.
■ Researchers with access to large radiologic image databases and substantial computational resources are encouraged to train foundation models using self-supervised learning (ie, without labels generated by humans) and to publish these pretrained models.
■ This sharing will enable researchers with limited resources to finetune these pretrained models using even small cohorts, thereby democratizing the field and fostering the exploration of rare condition
Weakly Supervised Deep Learning in Radiology
Leo Misera • Gustav Müller-Franzes• Daniel Truhn• Jakob Nikolas Kather
Radiology 2024; 312(1):e232085 - “Most of the DL models currently in use in clinical practice were trained in a supervised manner. This method uses large data sets with carefully crafted reference standard labels. For example, using supervised learning to train a model to segment malignant lesions on screening mammograms requires a large database of mammograms in which radiologists have annotated the precise position of every malignant lesion. The advantages of this training paradigm are that it has been proven to work well and that DL model performance scales with the availability of annotated data. However, the major disadvantage is that the construction of such databases for every radiologic use case can be prohibitively expensive when one takes into account the time required to manually label each radiologic image and the substantial median salary of radiologists .”
Weakly Supervised Deep Learning in Radiology
Leo Misera • Gustav Müller-Franzes• Daniel Truhn• Jakob Nikolas Kather
Radiology 2024; 312(1):e232085 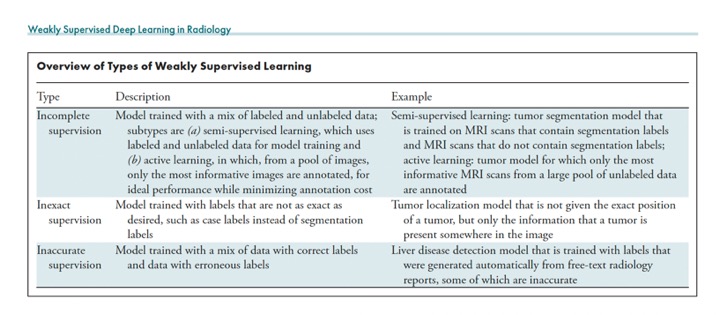
Weakly Supervised Deep Learning in Radiology
Leo Misera • Gustav Müller-Franzes• Daniel Truhn• Jakob Nikolas Kather
Radiology 2024; 312(1):e232085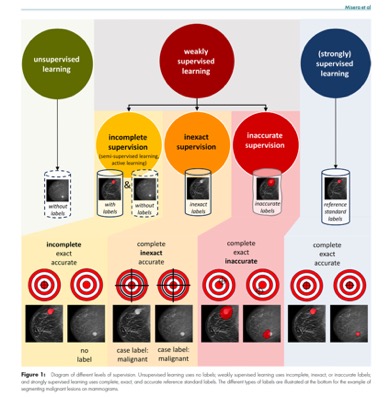
Weakly Supervised Deep Learning in Radiology
Leo Misera • Gustav Müller-Franzes• Daniel Truhn• Jakob Nikolas Kather
Radiology 2024; 312(1):e232085
- ”But the richness of medical images imperceptible to human eyes does not stop at the chest x-ray. For digital eyes, a chest CT encodes data for coronary artery disease risk with imputation of coronary calcium scores. One AI model has shown its superiority compared with radiologists for detecting pancreatic cancer from either chest CT or abdominal CT scans. Research is also assessing whether AI models and abdominal CTs can detect diabetes and predict cardiovascular risk. Beyond CT scans, mammography has information about risk of heart disease via presence of breast artery calcification.”
AI-enabled opportunistic medical scan interpretation.
Topol EJ.
Lancet. 2024 May 11;403(10439):1842. doi: 10.1016/S0140-6736(24)00924-3. PMID: 38735291. - ”Machine digital eyes can glean far more information from a scan than human experts can readily detect or accurately decipher. It may not be long before a chest x-ray report comes back with your risk of heart attack and stroke over the next decade, your coronary calcium score, your heart ejection fraction, the presence of leaky valve, and whether you have type 2 diabetes. Developments in artificial intelligence (AI) make this scenario likely in the coming years. This advance is an unanticipated, opportunistic output of deep learning AI.”
AI-enabled opportunistic medical scan interpretation.
Topol EJ.
Lancet. 2024 May 11;403(10439):1842. doi: 10.1016/S0140-6736(24)00924-3. PMID: 38735291. - ”The tip-off for much of this research came initially from use of AI tools with retinal images. Using AI, risks for some diseases that ophthalmologists cannot see—eg, risk of Parkinson’s disease, Alzheimer’s disease, hepatobiliary disease, kidney disease, heart attack, or stroke—became apparent. Similarly, what cardiologists cannot detect from an electrocardiogram—eg, risk of atrial fibrillation, heart attack, stroke, diabetes, kidney disease, anaemia, and filling pressure of the left heart—have been reported with AI models. Yet up to this point little has been implemented in the clinic to make use of such opportunistic outputs of medical images.”
AI-enabled opportunistic medical scan interpretation.
Topol EJ.
Lancet. 2024 May 11;403(10439):1842. doi: 10.1016/S0140-6736(24)00924-3. PMID: 38735291. - ”Despite this progress, a cautionary note is needed about AI-enabled opportunistic medical scan interpretation. A major concern is that the bonus outputs of medical images will be wrong, leading to unnecessary further evaluations, costs, and unwarranted patient anxiety. Before exploiting the unexpected windfall of information from medical scans, researchers need to fine-tune foundation AI models and rigorously prospectively assess the AI performance in diverse populations and settings. But looking ahead, there is great potential for what might be in store for AI’s medical discernibility. A prodigious amount of medical information is not currently being harnessed, much of which could be informative to patients and reduce the costs of additional medical imaging and missed diagnoses.”
AI-enabled opportunistic medical scan interpretation.
Topol EJ.
Lancet. 2024 May 11;403(10439):1842. doi: 10.1016/S0140-6736(24)00924-3. PMID: 38735291.
- “Medical image segmentation is a critical component in clinical practice, facilitating accurate diagnosis, treatment planning, and disease monitoring. However, existing methods, often tailored to specific modalities or disease types, lack generalizability across the diverse spectrum of medical image segmentation tasks. Here we present MedSAM, a foundation model designed for bridging this gap by enabling universal medical image segmentation. The model is developed on a large-scale medical image dataset with 1,570,263 image-mask pairs, covering 10 imaging modalities and over 30 cancer types. We conduct a comprehensive evaluation on 86 internal validation tasks and 60 external validation tasks, demonstrating better accuracy and robustness thanmodality-wise specialist models. By delivering accurate and efficient segmentation across a wide spectrum of tasks, MedSAM holds significant potential to expedite the evolution of diagnostic tools and the personalization of treatment plans.”
Segment anything in medical images
Jun Ma, Yuting He, Feifei Li , LinHan, Chenyu You ,Bo Wang
Nature Communications | ( 2024)1 5:654 - There is a growing demand for universal models inmedical image segmentation: models that can be trained once and then applied to a wide range of segmentation tasks. Suchmodels would not only exhibit heightened versatility in terms of model capacity but also potentially lead to more consistent results across different tasks. However, the applicability of the segmentation foundation models (e.g., SAM7) to medical image segmentation remains limited due to the significant differences between natural images and medical images. Essentially, SAM is a promptable segmentation method that requires points or bounding boxes to specify the segmentation targets. This resembles conventional interactive segmentation methods but SAM has better generalization ability, while existing deep learning-based interactive segmentation methods focus mainly on limited tasks and image modalities.
Segment anything in medical images
Jun Ma, Yuting He, Feifei Li , LinHan, Chenyu You ,Bo Wang
Nature Communications | ( 2024)1 5:654 - “We introduce MedSAM, a deep learning-powered foundation model designed for the segmentation of awide array of anatomical structures and lesions across diverse medical imaging modalities. MedSAM is trained on a meticulously assembled large-scale dataset comprised of over one million medical image-mask pairs. Its promptable configuration strikes an optimal balance between automation and customization,rendering MedSAMa versatile tool for universal medical image segmentation.”
Segment anything in medical images
Jun Ma, Yuting He, Feifei Li , LinHan, Chenyu You ,Bo Wang
Nature Communications | ( 2024)1 5:654 - “Considering these challenges, we argue that a more practical approach is to develop a promptable 2D segmentation model. The model can be easily adapted to specific tasks based on user-provided prompts, offering enhanced flexibility and adaptability. It is also able to handle both 2D and 3D images by processing 3D images as a series of 2D slices. Typical user prompts include points and bounding boxes andwe showsome segmentation examples with the different prompts. . It can be found that bounding boxes provide a more unambiguous spatial context for the region of interest, enabling the algorithm to more precisely discern the target area. This stands in contrast to point-based prompts, which can introduce ambiguity, particularly when proximate structures resemble each other. Moreover, drawing a bounding box is efficient, especially in scenariosinvolving multi-object segmentation. We follow the network architecture in SAM, including an image encoder, a prompt encoder, and a mask decoder. The image encoder maps the input image into a high-dimensional image embedding space.”
Segment anything in medical images
Jun Ma, Yuting He, Feifei Li , LinHan, Chenyu You ,Bo Wang
Nature Communications | ( 2024)1 5:654 - “Despite recent advancements in machine learning (ML) applications in health care, there have been few benefits and improvements to clinical medicine in the hospital setting. To facilitate clinical adaptation of methods in ML, this review proposes a standardized framework for the step-by-step implementation of artificial intelligence into the clinical practice of radiology that focuses on three key components: problem identification, stakeholder alignment, and pipeline integration. A review of the recent literature and empirical evidence in radiologic imaging applications justifies this approach and offers a discussion on structuring implementation efforts to helpother hospital practices leverage ML to improve patient care.”
Strategies for Implementing Machine Learning Algorithms in the Clinical Practice of Radiology
Allison Chae, et al.
Radiology 2024; 310(1):e223170 - Essentials
■ Real-world, representative data acquisition enables effective machine learning (ML).
■ Radiologists and other key stakeholders must work together for iterative and clinically informed use cases for ML in radiology.
■ Clinical implementation strategies involve the coordination of multidisciplinary teams and alignment with key institutional values.
■ Effectively characterizing the success of implementation of artificial intelligence in the clinical practice of radiology requires using user-centered metrics in real-world environments integrated with existing software and clinical workflows.
Strategies for Implementing Machine Learning Algorithms in the Clinical Practice of Radiology
Allison Chae, et al.
Radiology 2024; 310(1):e223170
- “Artificial intelligence (AI) and machine learning (ML) are poised to transform the way health care is delivered. AI is the use of computers to simulate intelligent tasks typically performed by humans. ML is a domain of AI that involves computers automatically learning from data without a priori programming. While AI has been critiqued as being in its “hype cycle” (throughout this article, AI will be used as shorthand for AI and ML), over time, it is likely that every medical specialty will be influenced by AI, and some will be transformed. As AI takes on a larger role in clinical practice, it is clear that multiple levels of oversight are needed. However, even with appropriate outside oversight, the importance of clinician review and trust of these technologies cannot be overstated.”
Preparing Clinicians for a Clinical World Influenced by Artificial Intelligence
James CA, Wachter RM, Woolliscroft JO
JAMA March 2022 doi:10.1001/jama.2022.3580 - "Importantly, equipping clinicians with the skills, resources, and support necessary to use AI-based technologies is now recognized as essential to successful deployment of AI in health care. To do so, clinicians need to have a realistic understanding of the potential uses and limitations of medical AI applications. Overlooking this fact risks clinician cynicism and suboptimal patient outcomes.”
Preparing Clinicians for a Clinical World Influenced by Artificial Intelligence
James CA, Wachter RM, Woolliscroft JO
JAMA March 2022 doi:10.1001/jama.2022.3580 - "Despite relatively weak evidence supporting the use of AI in routine clinical practice health care settings, AI models continue to be marketed and deployed. A recent example is the Epic Sepsis Model. While this model was widely implemented in hundreds of US hospitals, a recent study showed that it performed significantly worse in correctly identifying patients with early sepsis and improving patient outcomes in a clinical set- ting compared with performance observed during development of the model.”
Preparing Clinicians for a Clinical World Influenced by Artificial Intelligence
James CA, Wachter RM, Woolliscroft JO
JAMA March 2022 doi:10.1001/jama.2022.3580 - "At about the time that the EBM movement was being launched, a parallel movement began promoting shared decision-making between patients and clinicians. As AI-based predictions and algorithms continue to inform medical decisions, patients and clinicians must rethink shared decision-making as decisions may well now involve a new member of the team—an AI-derived algorithm. Ultimately, clinicians will bear much of the responsibility to successfully broker the triadic relationship between patients, the computer, and themselves.”
Preparing Clinicians for a Clinical World Influenced by Artificial Intelligence
James CA, Wachter RM, Woolliscroft JO
JAMA March 2022 doi:10.1001/jama.2022.3580 - "Ultimately, clinicians will bear much of the responsibility to successfully broker the triadic relationship between patients, the computer, and themselves. Clinicians will need to explain the role that AI has in their reasoning and recommendations. Over time, this relationship is likely to change, with the possibility of some decisions being made directly by patients and families based on AI recommenda- tions, bypassing the clinician. Navigating this transition—and finding the appropriate role for credentialed experts in it—will be a significant challenge in a health care system transformed by AI.”
Preparing Clinicians for a Clinical World Influenced by Artificial Intelligence
James CA, Wachter RM, Woolliscroft JO
JAMA March 2022 doi:10.1001/jama.2022.3580 - "AI will soon become ubiquitous in health care. Building on lessons learned as implementation strategies continue to be devised, it will be essential to consider the key role of clinicians as end users of AI-developed algorithms, processes, and risk predictors. It is imperative that clinicians have the knowledge and skills to assess and determine the appropriate application of AI outputs, for their own clinical practice and for their patients. Rather than being replaced by AI, these new technologies will create new roles and responsibilities for clinicians.”
Preparing Clinicians for a Clinical World Influenced by Artificial Intelligence
James CA, Wachter RM, Woolliscroft JO
JAMA March 2022 doi:10.1001/jama.2022.3580
- “InterpretML is an open-source Python package which exposes machine learning interpretability algorithms to practitioners and researchers. InterpretML exposes two types of interpretability – glassbox, which are machine learning models designed for interpretability (ex: linear models, rule lists, generalized additive models), and blackbox explainability techniques for explaining existing systems (ex: Partial Dependence, LIME). The package enables practitioners to easily compare interpretability algorithms by exposing multiple methods under a unified API, and by having a built-in, extensible visualization platform. InterpretML also includes the first implementation of the Explainable Boosting Machine, a powerful, interpretable, glassbox model that can be as accurate as many blackbox models.”
InterpretML: A Unified Framework for Machine Learning Interpretability
Harsha Nori et al.
arXiv Sept 2019 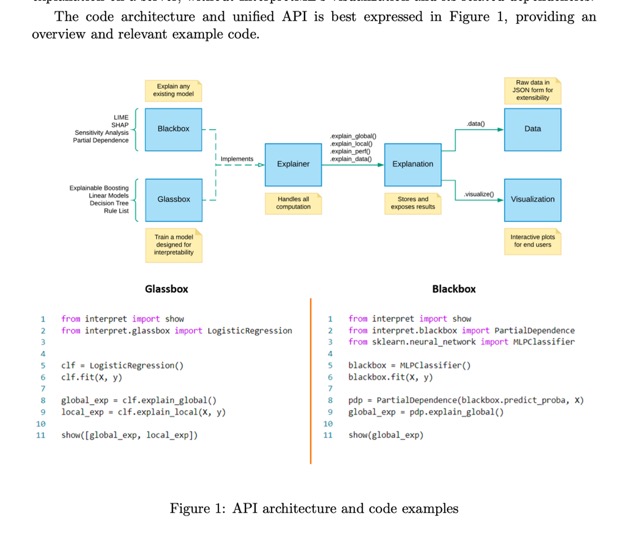
- Explainable Boosting Machine (EBM)
EBMs are highly intelligible, because the contribution of each feature to a final prediction can be visualized and understood by plotting fj. Because EBM is an additive model, each feature contributes to predictions in a modular way that makes it easy to reason about the contribution of each feature to the prediction. 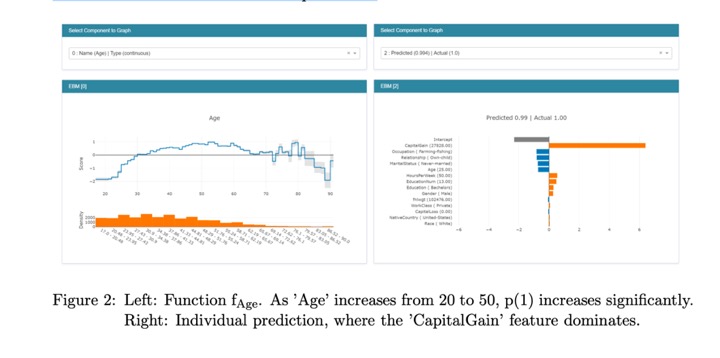
- In terms of predictive power, EBM often performs surprisingly well, and is comparable with state of the art methods like Random Forest and XGBoost.1 To keep the individual terms additive, EBM pays an additional training cost, making it somewhat slower than sim- ilar methods. However, because making predictions involves simple additions and lookups inside of the feature functions fj, EBMs are one of the fastest models to execute at prediction time. EBM’s light memory usage and fast predict times makes it particularly attractive for model deployment in production.
- "Most computer-based algorithms in medicine are "expert systems" — rule sets encoding knowledge on a given topic, which are applied to draw conclusions about specific clinical scenarios, such as detecting drug interactions or judging the appropriateness of obtaining imaging. Expert systems work the way an ideal medical student would: they take general principles about medicine and apply them to new patients."
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine
Obermeyer Z, Emanuel EJ
N Engl J Med 375;13 September 29, 2016 - "Machine learning, conversely, approaches problems as a doctor progressing through residency might: by learning rules from data. Starting with patient-level observations, algorithms sift through vast numbers of variables, looking for combinations that reliably predict outcomes."
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine
Obermeyer Z, Emanuel EJ
N Engl J Med 375;13 September 29, 2016 - "But where machine learning shines is in handling enormous numbers of predictors — sometimes, remarkably, more predictors than observations — and combining them in nonlinear and highly interactive ways.This capacity al- lows us to use new kinds of data, whose sheer volume or complexity would previously have made analyzing them unimaginable."
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine
Obermeyer Z, Emanuel EJ
N Engl J Med 375;13 September 29, 2016 - "Another key issue is the quantity and quality of input data. Machine learning algorithms are highly data hungry, often requiring millions of observations to reach acceptable performance levels. In addition, biases in data collection can substantially affect both performance and generalizability. Lactate might be a good predictor of the risk of death, for example, but only a small, nonrepresentative sample of patients have their lactate levels checked."
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine
Obermeyer Z, Emanuel EJ
N Engl J Med 375;13 September 29, 2016 - "Machine learning has become ubiquitous and indispensable for solving complex problems in most sciences. In astronomy, algorithms sift through millions of images from telescope surveys to classify galaxies and find supernovas."
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine
Obermeyer Z, Emanuel EJ
N Engl J Med 375;13 September 29, 2016 - "Increasingly, the ability to transform data into knowledge will disrupt at least three areas of medicine. First, machine learning will dramatically improve the ability of health professionals to es- tablish a prognosis. Current prognostic models (e.g., the Acute Physiology and Chronic Health Evaluation [APACHE] score and the Sequential Organ Failure Assessment [SOFA] score) are restricted to only a handful of variables, because humans must enter and tally the scores. But data could instead be drawn directly from EHRs or claims databases, allow- ing models to use thousands of rich predictor variables."
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine
Obermeyer Z, Emanuel EJ
N Engl J Med 375;13 September 29, 2016 - "Second, machine learning will displace much of the work of radiologists and anatomical pathologists. These physicians focus largely on interpreting digitized images, which can easily be fed directly to algorithms instead. Massive imaging data sets, com- bined with recent advances in computer vision, will drive rapid improvements in performance, and machine accuracy will soon exceed that of humans. Indeed, radiology is already partway there: algorithms can replace a second radiologist reading mammograms and will soon exceed human accuracy."
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine
Obermeyer Z, Emanuel EJ
N Engl J Med 375;13 September 29, 2016 - "The patient- safety movement will increasingly advocate the use of algorithms over humans — after all, algorithms need no sleep, and their vigilance is the same at 2 a.m. as at 9 a.m. Algorithms will also monitor and interpret streaming physiological data, replacing aspects of anesthesiology and criti- cal care. The time scale for these disruptions is years, not decades."
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine
Obermeyer Z, Emanuel EJ
N Engl J Med 375;13 September 29, 2016 - "Machine learning will become an indispensable tool for clinicians seeking to truly understand their patients. As patients’ conditions and medical technologies become more complex, the role of machine learning will grow, and clinical medicine will be challenged to grow with it."
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine
Obermeyer Z, Emanuel EJ
N Engl J Med 375;13 September 29, 2016 - "As in other industries, this challenge will create winners and losers in medicine. But we are optimistic that patients, whose lives and medical histories shape the algorithms, will emerge as the biggest winners as machine learning transforms clinical medicine."
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine
Obermeyer Z, Emanuel EJ
N Engl J Med 375;13 September 29, 2016
- ”ML algorithms evolve as they are exposed to more data. Nearly all ML algorithms used to analyze the pixel data of radiology examinations ‘‘learn’’ to give a specific answer by evaluating a large number of exams that have been hand-labeled. For example, a ML algorithm to detect lung nodules on chest radiographics will be trained by analyzing thousands of chest radiographs that humans have labeled as being normal, or as having nodules in the lungs.”
Canadian Association of Radiologists White Paper on Artificial Intelligence in Radiology An Tang et al. Canadian Association of Radiologists Journal 69 (2018) 120e135 - ” Representation learning refers to a subtype of ML in which no ‘‘hand-crafted’’ features are provided. Instead, the computer algorithm learns the features required to classify the provided data. The amount of training data has an impact on the performance of ML algorithms: adding data generally improves performance .If provided enough training data, systems based on representation learning may achieve better performance than traditional ML systems that incorporate ‘‘hand-crafted’’ features.”
Canadian Association of Radiologists White Paper on Artificial Intelligence in Radiology An Tang et al. Canadian Association of Radiologists Journal 69 (2018) 120e135 - “ML algorithms evolve as they are exposed to more data. Nearly all ML algorithms used to analyze the pixel data of radiology examinations ‘‘learn’’ to give a specific answer by evaluating a large number of exams that have been hand-labeled. For example, a ML algorithm to detect lung nodules on chest radiographics will be trained by analyzing thousands of chest radiographs that humans have labeled as being normal, or as having nodules in the lungs.
.”
Canadian Association of Radiologists White Paper on Artificial Intelligence in Radiology An Tang et al. Canadian Association of Radiologists Journal 69 (2018) 120e135 - “Neural networks are the algorithms that are most commonly used for image analysis today. The name refers to their design inspired by neurons in a brain. These neural networks are composed of layers of connected nodes (or neurons) and may contain thousands to millions of nodes. Each node receives information from some pattern of other nodes. If the information that node receives crosses a threshold, that node then sends a signal out to other groups of nodes. These outputs are weighted, in that they send a small signal out when they are weakly stimulated, and a strong signal when they receive appropriate input.”
Canadian Association of Radiologists White Paper on Artificial Intelligence in Radiology An Tang et al. Canadian Association of Radiologists Journal 69 (2018) 120e135 - “The learning process may occur either via supervised learning, in which a training set of data contains annotations by humans to match the desired output of the algorithm, or unsupervised learning, in which the training data do not contain annotations and the algorithm seeks to cluster or organize the data to reveal underlying patterns.”
Canadian Association of Radiologists White Paper on Artificial Intelligence in Radiology An Tang et al. Canadian Association of Radiologists Journal 69 (2018) 120e135 - “Almost all ML for analysis of radiology exams is currently performed via supervised learning which requires appropriately labeled training data. This highlights 2 challenges: 1) adequate labeling of key imaging find- ings, a tedious and time-consuming process, and 2) appropriate definition of ground truth (eg, radiology report, pathology report, clinical outcomes). Proper training of ML algorithms will require new ways to label data or to deal with loosely labeled data. Ground truth, which is often found on a continuum (eg, ranging from normal, probably normal, indeterminate, probably abnormal to definitely abnormal) may require artificial clustering into normal vs abnormal.”
Canadian Association of Radiologists White Paper on Artificial Intelligence in Radiology An Tang et al. Canadian Association of Radiologists Journal 69 (2018) 120e135 - “ML algorithms often require a large amount of data to ‘‘learn’’ to provide useful answers, and processing these data requires significant computing power. The rapid in- crease in power of graphical processing units (GPUs), initially created for accelerating computer graphics, such as used in gaming, have provided flexible hardware for ML purpose. The access to computational power and large training datasets has made these algorithms cost effective.”
Canadian Association of Radiologists White Paper on Artificial Intelligence in Radiology An Tang et al. Canadian Association of Radiologists Journal 69 (2018) 120e135 - “Therefore, it would be necessary to create freely available, modality-specific sandboxes for computer scientists to get a ‘‘feel’’ for the data, which will inform the design process. For example, in the computer vision community, the publicly available ImageNet dataset has been used to produce numerous breakthroughs in machine learning for image recognition, but many of these advances are not directly applicable to medical imaging.”
Canadian Association of Radiologists White Paper on Artificial Intelligence in Radiology An Tang et al. Canadian Association of Radiologists Journal 69 (2018) 120e135 - “For many applications, expert annotations (ie, measurements, contouring, and descriptions) for training AI algorithms are very expensive to obtain. A direction of research should be to make the annotation process more efficient, which can also be done with AI. The Medical Image Computing and Computer Assisted Intervention Workshop on Large-scale Annotation of Biomedical data and Expert Label Synthesis is an example of an academic venue with this research focus.”
Canadian Association of Radiologists White Paper on Artificial Intelligence in Radiology An Tang et al. Canadian Association of Radiologists Journal 69 (2018) 120e135 - “Development and validation of AI applications for radiology will require new thinking and approaches as it relates to collaborations and intellectual property between academic research laboratories and industrial partners. Several questions may arise in the process: 1) Who owns the data and intellectual property on the models developed jointly? 2) Should data sharing agreements be signed with individual sites or with the research consortium in the case of multicentre studies.”
Canadian Association of Radiologists White Paper on Artificial Intelligence in Radiology An Tang et al. Canadian Association of Radiologists Journal 69 (2018) 120e135
- “By now, it’s almost old news: big data will transform medicine. It’s essential to remember, however, that data by themselves are useless. To be useful, data must be analyzed, interpreted, and acted on. Thus, it is algorithms —
not data sets — that will prove transformative.
" Predicting the Future — Big Data, Machine Learning, and Clinical Medicine Obermeyer Z, Emanuel EJ N Engl J Med 375;13 September 29, 2016 - “Most computer-based algorithms in medicine are “expert systems” — rule sets encoding knowledge on a given topic, which are applied to draw conclusions about specific clinical scenarios, such as detecting drug interactions or judging the appropriateness of obtaining imaging. Expert systems work the way an ideal medical student would: they take general principles about medicine and apply them to new patients.”
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine Obermeyer Z, Emanuel EJ N Engl J Med 375;13 September 29, 2016 - “Machine learning, conversely, approaches problems as a doctor progressing through residency might: by learning rules from data. Starting with patient-level observations, algorithms sift through vast numbers of variables, looking for combinations that reliably predict outcomes.”
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine Obermeyer Z, Emanuel EJ N Engl J Med 375;13 September 29, 2016 - “But where machine learning shines is in handling enormous numbers of predictors — sometimes, remarkably, more predictors than observations — and combining them in nonlinear and highly interactive ways.This capacity al- lows us to use new kinds of data, whose sheer volume or complexity would previously have made analyzing them unimaginable.”
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine Obermeyer Z, Emanuel EJ N Engl J Med 375;13 September 29, 2016 - “Another key issue is the quantity and quality of input data. Machine learning algorithms are highly data hungry, often re- quiring millions of observations to reach acceptable performance levels.In addition, biases in data collection can substantially affect both performance and generalizability. Lactate might be a good predictor of the risk of death, for example, but only a small, nonrepresentative sample of patients have their lactate levels checked.”
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine Obermeyer Z, Emanuel EJ N Engl J Med 375;13 September 29, 2016 - “Machine learning has become ubiquitous and indispensable for solving complex problems in most sciences. In astronomy, algorithms sift through millions of images from telescope surveys to classify galaxies and find supernovas.”
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine Obermeyer Z, Emanuel EJ N Engl J Med 375;13 September 29, 2016 - “Increasingly, the ability to transform data into knowledge will disrupt at least three areas of medicine. First, machine learning will dramatically improve the ability of health professionals to es- tablish a prognosis. Current prognostic models (e.g., the Acute Physiology and Chronic Health Evaluation [APACHE] score and the Sequential Organ Failure Assessment [SOFA] score) are restricted to only a handful of vari- ables, because humans must enter and tally the scores. But data could instead be drawn directly from EHRs or claims databases, allow- ing models to use thousands of rich predictor variables.”
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine Obermeyer Z, Emanuel EJ N Engl J Med 375;13 September 29, 2016 - “Second, machine learning will displace much of the work of radiologists and anatomical pathologists. These physicians focus largely on interpreting digitized images, which can easily be fed directly to algorithms instead. Massive imaging data sets, com- bined with recent advances in computer vision, will drive rapid improvements in performance, and machine accuracy will soon exceed that of humans. Indeed, radiology is already partway there: algorithms can replace a second radiologist reading mammograms and will soon exceed human accuracy.”
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine Obermeyer Z, Emanuel EJ N Engl J Med 375;13 September 29, 2016 - “The patient- safety movement will increasingly advocate the use of algorithms over humans — after all, algorithms need no sleep, and their vigilance is the same at 2 a.m. as at 9 a.m. Algorithms will also monitor and interpret streaming physiological data, replacing aspects of anesthesiology and criti- cal care. The time scale for these disruptions is years, not decades.”
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine Obermeyer Z, Emanuel EJ N Engl J Med 375;13 September 29, 2016 - “Machine learning will become an indispensable tool for clinicians seeking to truly understand their patients. As patients’ conditions and medical technologies become more complex, the role of machine learning will grow, and clinical medicine will be challenged to grow with it.”
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine Obermeyer Z, Emanuel EJ N Engl J Med 375;13 September 29, 2016 - “As in other industries, this challenge will create winners and losers in medicine. But we are optimistic that patients, whose lives and medical histories shape the algorithms, will emerge as the biggest winners as machine learning transforms clinical medicine.”
Predicting the Future — Big Data, Machine Learning, and Clinical Medicine Obermeyer Z, Emanuel EJ N Engl J Med 375;13 September 29, 2016


