Imaging Pearls ❯ Colon ❯ Colon Cancer
|
-- OR -- |
|
- “Juvenile polyposis syndrome (JPS) is characterized by predisposition to hamartomatous polyps in the gastrointestinal (GI) tract, specifically in the stomach, small intestine, colon, and rectum. The term "juvenile" refers to the type of polyp rather than to the age of onset of polyps. Most individuals with JPS have some polyps by age 20 years; some may have only four or five polyps over their lifetime, whereas others in the same family may have more than 100. If the polyps are left untreated, they may cause bleeding and anemia. Most juvenile polyps are benign; however, malignant transformation can occur. Risk for GI cancers ranges from 11% to 86%. Most of this increased risk is attributed to colon cancer, but cancers of the stomach, upper GI tract, and pancreas have also been reported. ”
Juvenile Polyposis Syndrome.
Larsen Haidle J, MacFarland SP, Howe JR.
2003 May 13 [Updated 2022 Feb 3]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1469 - “The diagnosis of JPS is established in a proband with any one of the following features: More than five juvenile polyps of the colon or rectum Multiple juvenile polyps of the upper and lower gastrointestinal tract Any number of juvenile polyps and a family history of juvenile polyposis Identification of a heterozygous pathogenic (or likely pathogenic) variant in one of the genes listed in Table 1.”
Juvenile Polyposis Syndrome.
Larsen Haidle J, MacFarland SP, Howe JR.
2003 May 13 [Updated 2022 Feb 3]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1469 - “Individuals with JPS/HHT have variable findings of juvenile polyposis and HHT (see Table 2). Most individuals with JPS who have an SMAD4 germline pathogenic variant have one or more clinical features of HHT. The findings of HHT may manifest in early childhood. A high frequency of pulmonary arteriovenous malformations (with digital clubbing) and epistaxis has been consistently noted in individuals with SMAD4-related HHT. Conversely, telangiectases do not appear to be a constant feature. Additional complications reported in individuals with JPS/HHT include anemia, migraine headaches, and exercise intolerance.”
Juvenile Polyposis Syndrome.
Larsen Haidle J, MacFarland SP, Howe JR.
2003 May 13 [Updated 2022 Feb 3]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1469 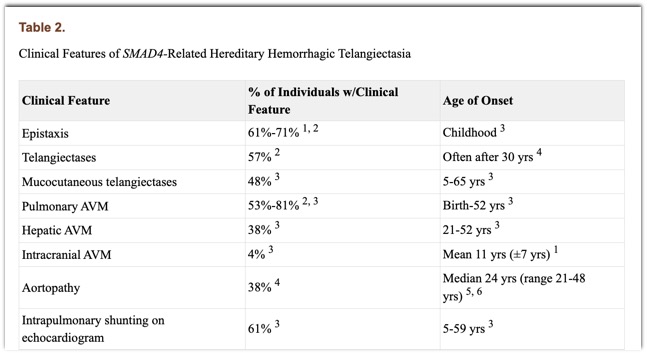
- CT Evaluation of the Colon: Pearls and Pitfalls
- Bowel wall thickening
- Increased submucosal fat
- Pneumatosis coli
- Bowel obstruction (i.e. tumor, volvulus)
- Hernia (external)
- Pericolonic inflammation (i.e. diverticulitis, perforation, appendix epipolicae) - CT of the Colon: Colonic Distension
- Rectal contrast
- Oral contrast (delayed scans at 3-4 hours)
- Virtual colonoscopy
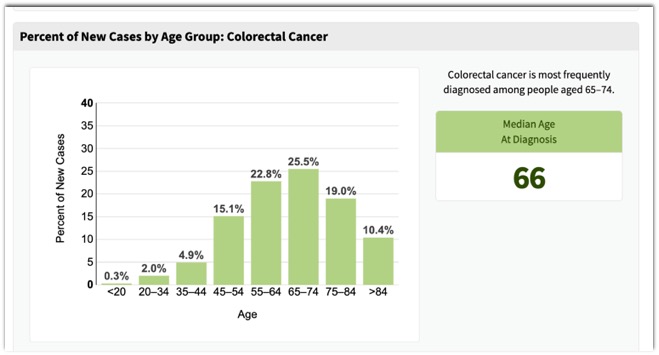
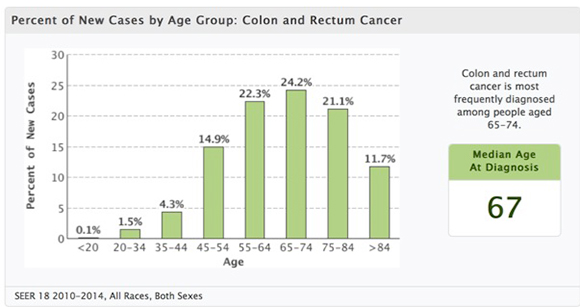
- “Colorectal cancer is a disease that is curable if detected early and preventable if precursor adenomas are detected and removed. Approximately 130,000 new cases were diagnosed in the United States in 2000, and approximately 56,000 deaths were attributed to the disease. The typical age at which most patients are diagnosed is during the sixth and seventh decades of life.”
Imaging in the Diagnosis, Staging, and Follow-Up of Colorectal Cancer Iyer RB et al. AJR 2002;179:3–13 - “Evolution in the treatment of metastatic colorectal cancer (mCRC) has led to significant improvement in the survival of these patients. Surgery is useful in patients with resectable disease. Liver-directed therapies such as hepatic arterial infusion, transarterial radio- and chemoembolization, and percutaneous ablation are sometimes used by oncologists when the liver is the only site of metastatic disease. Unresectable mCRC is typically treated with systemic chemotherapy.”
Update on the Role of Imaging in Management of Metastatic Colorectal Cancer Tirumani SH et al. RadioGraphics 2014; 34:1908–1928 - “CRC most commonly metastasizes to the liver, with more than one-half of patients developing hepatic metastases either synchronously or metachronously.The lung is the second most common organ to harbor CRC metastases, followed by the peritoneal cavity. Peritoneal involvement is seen as peritoneal carcinomatosis and, in some cases, as pseudomyxoma peritonei, especially with the primary tumor arising from the appendix. Brain and bone metastases are uncommon.”
Update on the Role of Imaging in Management of Metastatic Colorectal Cancer Tirumani SH et al. RadioGraphics 2014; 34:1908–1928 - “The most reliable phase for the detection of hepatic metastases is the portal venous phase (approximately 60–70 seconds following initiation of intravenous contrast material administration), with a detection rate of 85% and a positive predictive value of 96%. In the arterial phase, CRC metastases can have peripheral rimlike enhancement. Calcification is noted in 11% of CRC liver metastases.”
Update on the Role of Imaging in Management of Metastatic Colorectal Cancer Tirumani SH et al. RadioGraphics 2014; 34:1908–1928 - “Detection of liver metastases at multidetector CT can be difficult in the presence of fatty liver, which is most often the result of concurrent chemotherapy . Differentiation of small hemangiomas and cysts less than 1 cm in size from metastases can also be difficult at times due to volume averaging. The sensitivity of CT for detect- ing lesions less than 1 cm falls from 65%–95% to 31%–38%. Multidetector CT has a specificity of 67% in characterizing lesions as benign or malignant, compared with 81% for MR imaging.”
Update on the Role of Imaging in Management of Metastatic Colorectal Cancer Tirumani SH et al. RadioGraphics 2014; 34:1908–1928
- “Despite being readily preventable, colorectal cancer ranks second behind only lung cancer in overall mortality. However, this situation could be reversed if screening tests that effectively detect advanced adenomas and early cancers were broadly applied. Computed tomographic colonography (CTC) reflects an ideal balance of minimal invasiveness with high-level performance, assuming all facets of the examination are appropriately addressed. Unfortunately, this promising screening test remains grossly underused. This article details the technical and interpretive approaches used by one successful CTC screening program.”
Imaging and Screening for Colorectal Cancer with CT Colonography. Pickhardt PJ Radiol Clin North Am. 2017 Nov;55(6):1183-1196 - “Colorectal cancer (CRC) remains the second-leading cause of cancer death, despite the fact that it is readily preventable via screen detection (and removal) of clinically relevant polyps. This disconnect is primarily due to the fact that many adults are not screened, whereas others undergo only stool-based testing, which does not confer effective cancer prevention.Given the critical importance of cancer prevention beyond cancer detection alone, the American Cancer Society expressed a strong preference for the endoscopist and radiologic tests that provide for visualization of benign but potentially precancerous polyps in its landmark 2008 screening guidelines.”
Imaging and Screening for Colorectal Cancer with CT Colonography. Pickhardt PJ Radiol Clin North Am. 2017 Nov;55(6):1183-1196 - “As with bowel preparation, adequate luminal distension of the colon is critical to a successful CTC study. UWHC’s distention protocol has evolved and improved over time, resulting in inadequate segmental distention in less than 1% of cases.The use of automated low-pressure carbon dioxide (CO2) delivery is clearly preferred over manual room air insufflation.Nearly all early reports of perforation at CTC involved the use of manual staff-controlled room air insufflation in symptomatic patients, whereas the risk of perforation with automated CO2 delivery likely approaches zero for asymptomatic screening.With regard to both study quality (ie, degree of distention) and after-procedural discomfort, the author has shown that automated CO2 is superior to the manual room air technique.”
Imaging and Screening for Colorectal Cancer with CT Colonography. Pickhardt PJ Radiol Clin North Am. 2017 Nov;55(6):1183-1196
- Colon Polyps: Facts
• Polyps are common (60% men, 40% women at autopsy)
• Not all polyps are adenomas
• The prevalence of adenomas in the general population is 30-50% and increases with age
• Only 1-3% of all adenomas ever become malignant.
• It takes 10-15 years for an adenoma to become a carcinoma. - Colon Polyps : Who to Screen
• Estimated that approximately 12,000,000 adults per year in US are eligible for colorectal cancer screening
• High risk people at 40 (only represent 17% of patients who get colon cancer)
• High risk include FP, NFPCC, UC, Crohn, Family History
• Average risk people at 50 - Classic Colonoscopy: How Accurate is ir Really?
• Definitive procedure for evaluation of entire colon
– Requires sedation
– Risk of bleeding, perforation (1:1000), death (1-3:10,000)
• Completion rate 75-99% - anatomy & experience
– Polyps can be removed
• Misses 5-10% of adenomas > 1 cm.
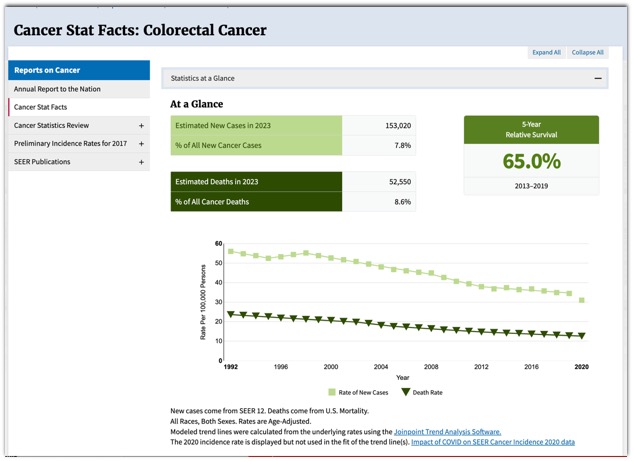
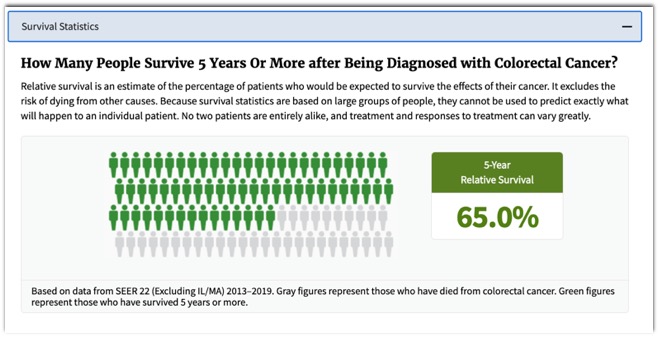
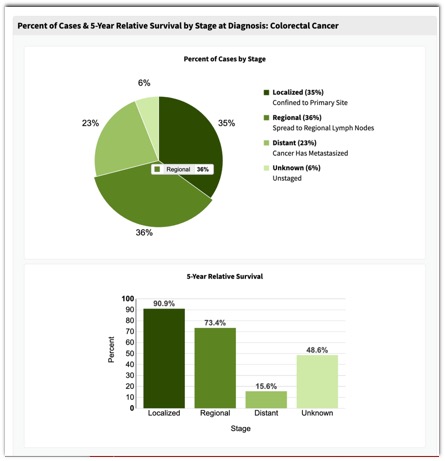
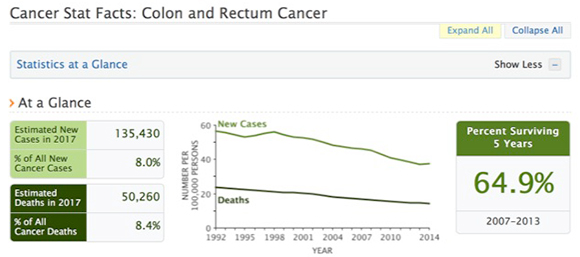
– Is it really necessary to remove tiny polyps? - SEER Data: Colon Cancer
- SEER Data: Colon Cancer
- BACKGROUND: Advanced neoplasia represents the primary target for colorectal-cancer screening and prevention. We compared the diagnostic yield from parallel computed tomographic colonography (CTC) and optical colonoscopy (OC) screening programs.
CONCLUSIONS: Primary CTC and OC screening strategies resulted in similar detection rates for advanced neoplasia, although the numbers of polypectomies and complications were considerably smaller in the CTC group. These findings support the use of CTC as a primary screening test before therapeutic OC. CT colonography versus colonoscopy for the detection of advanced neoplasia.
Kim DH et al. N Engl J Med. 2007 Oct 4;357(14):1403-12.
- Benign Colon Masses
- Inflammatory polyps (pseudopolyp)
- Hamartomas
- Juvenile polyps
- Lipomas
- Lymphoid polyps - Benign Colon Masses
- inflammatory polyps (pseudopolyp)
usually associated with chronic inflammatory bowel diseases such as Crohn's disease or ulcerative colitis
- hamartomas
contain normal cells that have an abnormal arrangement - Benign Colon Masses
- juvenile polyps
also called retention polyps that contain many mucous glands
usually occur as a single large polyp
mostly found in children under 10 years of agelipomas
develop within the fat cells in the colon
- lymphoid polyps
contain lymphoid cells, a type of white blood cell
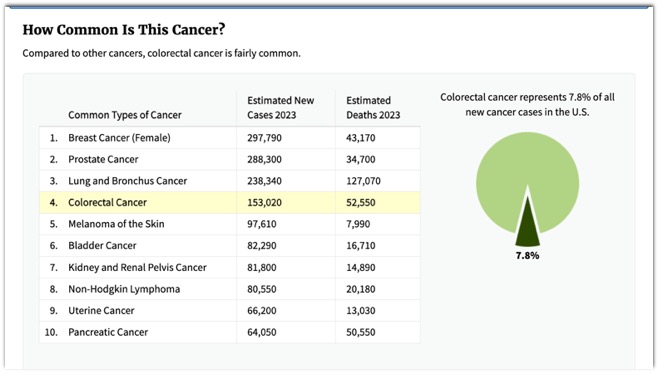
- Colon Cancer: Facts
- 3rd most common cancer in US
- 150,000 new cases each year in US
- > 1 million new cases diagnosed each year throughout the world
- 3nd most frequent cause of cancer death
- Over 56,000 deaths in US each year
- 90% cure rate when detected early
- Mortality has improved only slightly
- Screening participation is low. - Colon Polyps: Facts
- Most colorectal cancers arise from detectable precancerous lesions ---adenomatous polyps. (Adenoma-carcinoma sequence)
- Detection and removal of these polyps has been shown to prevent the development of colorectal cancer. - Colon Polyps: Facts
- Polyps are common (60% men, 40% women at autopsy)
- The prevalence of adenomas in the general population is 30-50% and increases with age
- Only 1-3% of all adenomas ever become malignant.
- Its takes 10-15 years for an adenoma to become a carcinoma. - Colon Polyps: Facts
- Size and malignancy
- polyp < 5 mm = 0%
- polyp 5 - 9 mm = <0.5%
- polyp 10 - 20 mm = 1-10%
- polyp > 20 mm = 40-50%
(Diminutive < 5mm, small 6-9mm, large > 10mm) - Colon Polyps: Screening
- Estimated that approximately 12,000,000 adults per year in US are eligible for colorectal cancer screening
- High risk people at 40 (only represent 17% of patients who get colon cancer)
- FP, NFPCC, UC, Crohn, Family History
- Average risk people at 50 - “ In 2008, the American Cancer Society guideline for colorectal cancer screening was revised jointly with the US Multi-Society Task Force on Colorectal Cancer. The ACS/ACR includes CTC every 5 years as an option for average risk patients.”
ACR Appropriateness Criteria on Colorectal Cancer Screening
Yee J et al.J Am Coll Radiol 2010;7:670-678 - ACS Guidelines
- Average risk people age 50
- Tests that detect cancer and polyps
- Flexible sigmoidoscopy every 5 years
- Colonoscopy every 10 years
- Double contrast BE every 5 years
- CT colonography every 5 years - Screening Choices
- Colonoscopy (q 10 yrs)
- Definitive procedure for evaluation of entire colon
- Requires sedation
- Small risk of bleeding, perforation (1:1000), death (1-3:10,000)
- Completion rate 75-99% depending on anatomy & experience
- Polyps can be removed
- Misses 5-10% of adenomas > 1 cm.
- “ Patients having rectal cancers with lymphovascular invasion showed a significantly increased mean superior hemorrhoidal vein diameter at presentation, which could be identified with pretreatment CT and help to direct the application of neoadjuvant treatment strategies.”
Prediction of Lymphovascular Invasion in Rectal cancer by Preoperative CT
Wu CC et al.
AJR 2013; 201:985-992 - “ The diameter of the superior hemorrhoidal vein was significantly higher in patients with lymphovascular invasion than in those without (mean diameter, 44 vs 30 mm, respectively; p<0.001) and was significantly higher in patients with distant metastases than in those without (p=0.044).”
Prediction of Lymphovascular Invasion in Rectal cancer by Preoperative CT
Wu CC et al.
AJR 2013; 201:985-992 - “Presence of lymphovascular invasion which is defined as cancer cells in peritumoral lymphatic vessels or small nonmuscularized blood vessels or both, has been recognized as an important prognostic determinant in colorectal cancer independent of stage.”
Prediction of Lymphovascular Invasion in Rectal cancer by Preoperative CT
Wu CC et al.
AJR 2013; 201:985-992 - “Although lymphovascular invasion in rectal cancer is considered a strong stage independent prognostic factor, our study show the pretreatment CT could be used to predict LVI with high sensitivity (75.8-85.2%), specificity (90.5-92.1%), and positive likelihood ratio (8.0-10.8) by using the diameter of superior hemorrhoidal vein with a cutoff value of 37 mm.”
Prediction of Lymphovascular Invasion in Rectal cancer by Preoperative CT
Wu CC et al.
AJR 2013; 201:985-992 - “Although lymphovascular invasion in rectal cancer is considered a strong stage independent prognostic factor, our study show the pretreatment CT could be used to predict LVI using the diameter of superior hemorrhoidal vein with a cutoff value of 37 mm.”
Prediction of Lymphovascular Invasion in Rectal cancer by Preoperative CT
Wu CC et al.
AJR 2013; 201:985-992 - “Prediction of poor prognostic features in rectal cancers has helped direct the application of neoadjuvant treatment strategies.”
Prediction of Lymphovascular Invasion in Rectal cancer by Preoperative CT
Wu CC et al.
AJR 2013; 201:985-992 - “ Carcinoid tumors of the gastrointestinal tract are a biologically heterogeneous group of tumors with a spectrum ranging from benign indolent tumors to aggressive metastatic malignancies.”
Imaging Features of Carcinoid Tumors of the Gastrointestinal Tract
Ganeshan D et al.
AJR 2013; 201:773-786 - Appendeceal Carcinoid Tumors: Facts
- Represent only 6% of NETs of the GI tract
- Usually small and incidental finding at pathology
- More common in younger patients
- Most common tumor of the appendix (60%) - Colorectal Neuroendocrine Tumors: facts
- Colonic NETs are usually > 2cm and involve cecum and ascending colon
- 34% of GI NETs occur in the rectum
- Synptoms range from pain to GI bleeding to weight loss although many patients are asymptomatic
- Nodal spread more common with larger tumors
- Colorectal Cancer: Facts
- Approximately 60% of patients with colorectal carcinoma eventually develop lymph node or distant metastases
- The liver is the initial site of metastases in 30% of cases
- Classic chemotherapy is cytotoxic (5-FU, leucovorin, irinotecan or oxaliplatin) and new chemotherapy is recombinant human monoclonal antibody against vascular endothelial growth factor (bevacizumab or Avastin)
- Avastin has improved survival of stage IV colon cancer but the agent inhibits tumor growth rather than shrinkage - “ Our retrospective study compared RECIST version 1.1 with modified CT criteria to determine their respective utilities as a prognostic indicator. Using both sets of criteria, we compared treatment responses of patients with colorectal liver metastases treated with bevacizumab-containing chemotherapy with those of patients treated with chemotherapy alone.”
Response Evaluation in Patients With Colorectal Liver Metastases: RECIST Version 1.1 Versus Modified CT Criteria
Chung WS et al.
AJR 2012; 199:809-815 - “Evaluating treatment response with tumor size and density changes on CT was a better predictor of time to tumor progression than changes in tumor size alone in both groups.”
Response Evaluation in Patients With Colorectal Liver Metastases: RECIST Version 1.1 Versus Modified CT Criteria
Chung WS et al.
AJR 2012; 199:809-815 - “In conclusion, response evaluation using changes in tumor size and density on CT better predicted time to tumor progression than changes in tumor size only in patients with colorectal liver metastases treated with bevacizumab containing chemotherapy or chemotherapy alone.”
Response Evaluation in Patients With Colorectal Liver Metastases: RECIST Version 1.1 Versus Modified CT Criteria
Chung WS et al.
AJR 2012; 199:809-815 - “On the basis of our results, we suggest adding tumor density changes to new criteria for evaluating treatment response of the anticancer treatment agents or tumor type. These new criteria should be tested further in large scale studies of other tumors treated with various therapies.”
Response Evaluation in Patients With Colorectal Liver Metastases: RECIST Version 1.1 Versus Modified CT Criteria
Chung WS et al.
AJR 2012; 199:809-815 - Measuring Changes in Tumor Attenuation
- Venous phase images (60-70 seconds)
- 2 ml/kg IV dose of Ultravist 300
- Key was drop in attenuation by 15% of the target lesion
- Note: scan protocols must be the same between studies
- “ As the cause of large bowel obstruction, colorectal cancer, diverticulitis, and volvulus account for approximately 80-85% of the cases of large bowel obstruction.”
Radiological diagnosis of large-bowel obstruction: neoplastic etiology
Hyakawa K et al.
Emerg Radiol (2013) 20:69-76 - “ Colorectal tumors are the most common cause of large bowel obstruction in about 60% of cases. Most of these tumors are adenocarcinoma and cause obstruction by luminal compromise and bowel wall thickening.”
Radiological diagnosis of large-bowel obstruction: neoplastic etiology
Hyakawa K et al.
Emerg Radiol (2013) 20:69-76
- Ulcerative Colitis and Colon Cancer: Facts
- Risk increases after disease present for 8-10 years and correlates with extent of disease
- Best estimates of risk are 5% after 10-20 years of disease and 9% per year thereafter
- Because biopsies are necessary in screening these patients for dysplasia CTC is not used but classic colonoscopy is needed - Colorectal Cancer: Risk Categories
- 1. average risk (age of 50 years old)
- 2. moderate risk (first degree relative with a history of adenoma or carcinoma or personal history of adenoma or carcinoma)
- 3. high risk (hereditary syndromes like familial polyposis, personal history of ulcerative colitis or Crohn’s disease) - Why do we do Colon Cancer screening?
- The prevalence of adenomas in the general population is 30-50% and increases with age
- The vast majority of adenomas are <1cm and these lesions have about a 1% likelihood of containing invasive cancer.
- Only 1-3% of adenomas ever progress to cancer
- Adenomas >1 cm have have a 10% chance of containing invasive cancer and a 25% chance of progressing to invasive cancer over 20 years
- Approximately 8% may undergo malignant degeneration within 10 years - ACR Appropriateness Criteria on Colorectal Cancer Screening: Summary Statements
- Computed tomographic colonography has emerged as the leading imaging technique for colorectal cancer screening
- The DCBE remains an imaging test that is also appropriate for colorectal cancer screening, particularly when CTC is not available
- Computed tomographic colonography is the preferred test after incomplete colonoscopy
- Imaging tests, including CTC and barium enema, are usually not appropriate for colorectal cancer screening in high risk patients with hereditary nonpolyposis colrectal cancer and inflammatory bowel disease - Ulcerative Colitis and Colon Cancer: Facts
- Risk increases after disease present for 8-10 years and correlates with extent of disease
- Best estimates of risk are 5% after 10-20 years of disease and 9% per year thereafter
- Because biopsies are necessary in screening these patients for dysplasia CTC is not used but classic colonoscopy is needed - Colorectal Cancer: Risk Categories
- 1. average risk (age of 50 years old)
- 2. moderate risk (first degree relative with a history of adenoma or carcinoma or personal history of adenoma or carcinoma)
- 3. high risk (hereditary syndromes like familial polyposis, personal history of ulcerative colitis or Crohn’s disease)
- Why do we do Colon Cancer screening?
- The prevalence of adenomas in the general population is 30-50% and increases with age
- The vast majority of adenomas are <1cm and these lesions have about a 1% likelihood of containing invasive cancer.
- Only 1-3% of adenomas ever progress to cancer








