Anastomotic Leak After Robot-Assisted Laparoscopic Radical Prostatectomy (RALRP): Evaluation with MDCT Cystography
Anastomotic Leak After Robot-Assisted Laparoscopic Radical Prostatectomy (RALRP): Evaluation with MDCT Cystography Satomi Kawamoto MD The Russell H. Morgan Department of Radiology and Radiological Science Department of Urology, Johns Hopkins Medical Institutions |
Introduction Robot-assisted laparoscopic radical prostatectomy is a minimally invasive prostate surgery for localized prostate cancer using the daVinci robotic system (Intuitive Surgical, Sunnyvale, Calif). There has been evolution of open radical prostatectomy to laparoscopic prostatectomy, to robot-assisted prostatectomy in recent years [1]. Robotic systems enhance surgeons technical abilities and offer the potential more precise surgical technique [2]. Since it was first described in 2001 [3, 4] robot-assisted radical prostatectomy is gaining acceptance and popularity among urologists [5]. |
Introduction Robot-assisted laparoscopic radical prostatectomy provides several advantages over open and laparoscopic prostatectomy such as precise dissection through improved instrument control with articulating tips, 3-D vision and magnified view, intuitive eye-hand coordination, motion scaling and filter of tremor [5]. With laparoscopic approaches, a hospital stay of only 1 day has become routine in many centers [6]. Good results with urinary continence and potency after robot-assisted laparoscopic radical prostatectomy have been reported [7, 8], although whether continence and/or potency are better with a laparoscopic/robotic-assisted approach compared with open surgery in expert hands is uncertain [1]. Surgical margins are comparable to those with open approaches [9] and are partly influenced by surgical technique and experience [1]. |
Introduction For laparoscopic radical prostatectomy, the majority of complications are urologic. The most common complication is anastomotic leak which occurred in approximately 10% of patients [10]. Following robot-assisted laparoscopic radical prostatectomy, the reported incidence of postoperative urethrovesical anastomotic urinary leak was 8.6 to 13.6% [11, 12], and it can be associated with intraperitoneal leak when transperitoneal approach is used. |
Introduction The purposes of this exhibit are to analyze and illustrate the pattern of anastomotic leak after robot-assisted laparoscopic radical prostatectomy on MDCT cystography and to review and illustrate key surgical techniques of robot-assisted laparoscopic radical prostatectomy to explain the mechanism of the intraperitoneal urine leak. Incidence and clinical significance of anastomotic leak after robot-assisted laparoscopic radical prostatectomy were also reviewed. |
Key surgical technique of robot-assisted laparoscopic radical prostatectomy and mechanisms of intraperitoneal urine leak The da Vinci surgical system
|
Transperitoneal and extraperitoneal approach There are two approaches of robot-assisted laparoscopic radical prostatectomy; (1) transperitoneal and (2) extraperitoneal approaches. For transperitoneal approach, pneumoperitoneum is initially achieved, and a port and the laparoscope are introduced for initial abdominal inspection. Four additional trocars are then placed. The extraperitoneal space - Retizius (retropubic) space is entered through an inverted U-shaped incision in the parietal peritoneum superior to the bladder to include the urachus and laterally to medial umbilical ligaments. With the Montsouris technique [4], the vas deference and seminal vesicles are dissected retrovesically through an incision of the peritoneum overlying the vas deferens before further dissection of the prostate gland. With this technique, two routes of interaperitoneal communication (anterior to the bladder, and posterior to the bladder) from the uretrovesical anastomosis are made. Alternative to Montsouris technique, the seminal vesicles may be dissected after separation of the prostate from the posterior bladder neck. |
Transperitoneal and extraperitoneal approach
|
Transperitoneal and extraperitoneal approach The use of transperitoneal or extraperitoneal approach for laparoscopic or robot-assisted laparoscopic prostatectomy is largely a matter of surgeon preference, and there is no consistently demonstrated advantage for either approach [1]. However, extraperitoneal approach helps to confine any urine leak that may occur from the vesicourethral anastomosis within the extraperitoneal space [1]. |
CT CYSTOGRPHIC FINDINGS OF ANASTOMOTIC LEAK CT cystography technique
|
CT CYSTOGRPHIC FINDINGS OF ANASTOMOTIC LEAK Extraperitoneal anastomotic leak
|
CT CYSTOGRPHIC FINDINGS OF ANASTOMOTIC LEAK 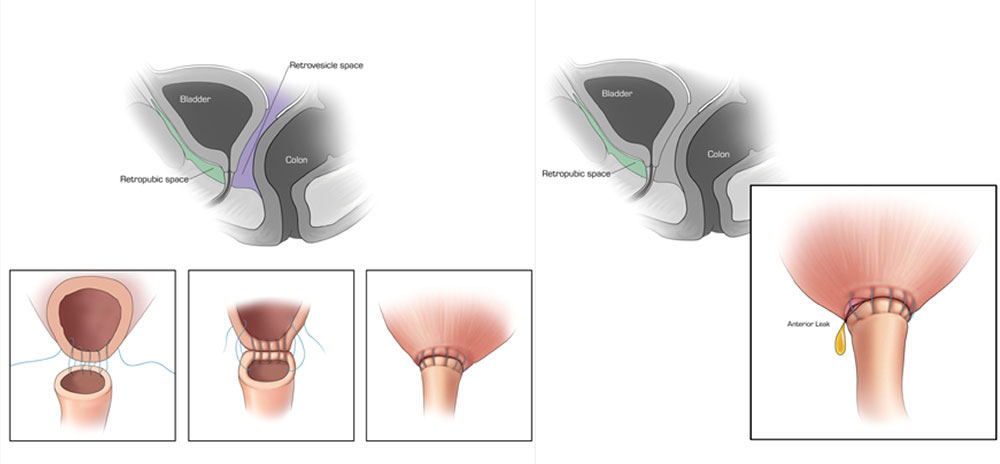 Extraperitoneal anastomotic leak Left: Illustration depicting sagittal section through pelvis, showing retrovesicle and tretropubic space(A). Illustrations, (1, 2, 3.) showing vesicourethral anastomosis. Right: Illustration depicting anterior leak at vesicourethral anastomosis. Sagittal section shows leak contained in the extraperitoneal space. |
CT CYSTOGRPHIC FINDINGS OF ANASTOMOTIC LEAK 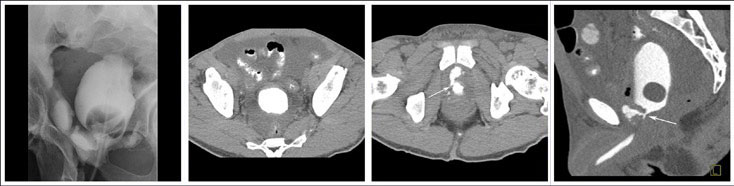 Extraperitoneal anastomotic leak Figure 1. Status post robot-assisted laparoscopic prostatectomy for prostate cancer (pT2bN0Mx, Gleason 7 adenocarcinoma). (a) Fluoroscopic cystogram was performed 13 days after surgery because of recurrent postoperative ileus. It demonstrates moderate extraperitoneal leak within the pelvis. No clear intraperitoneal leak is detected. (b) Axial CT csytogram at the superior aspect of the bladder obtained 4 weeks after surgery shows moderate ascites within the pelvis. (c) Axial and (d) sagittal MPR CT cystograms obtained 4 weeks after surgery show persistent but decrease in size of extraperitoneal leak from the anterior aspect of the anastomosis. The patient was readmitted and treated conservatively. It was felt that undetected intraperitoneal leak that was not detected on the initial cystogram may have lead to a peritonitis causing a recurrent ileus. |
CT CYSTOGRPHIC FINDINGS OF ANASTOMOTIC LEAK 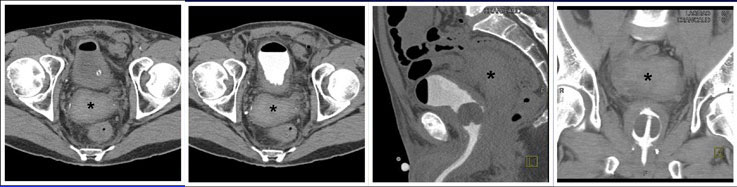 Extraperitoneal anastomotic leak Figure 2. Status post robot-assisted laparoscopic radical prostatectomy (pT2bNxMx, Gleason 7 adenocarcinoma). The patient experienced episodes of leaking urine around Foley catheter with no drainage via Foley catheter. CT cystography obtained 12 days after surgery. (a) Axial noncontrast CT shows moderate sized radiodense fluid collection posterior to the bladder, consistent with hematoma (asterisk). (b) Axial, (c) lateral, and (d) anterior volume rendered CT cystograms show thick-walled bladder, but there is no evidence of anastomotic leak. |
CT CYSTOGRPHIC FINDINGS OF ANASTOMOTIC LEAK 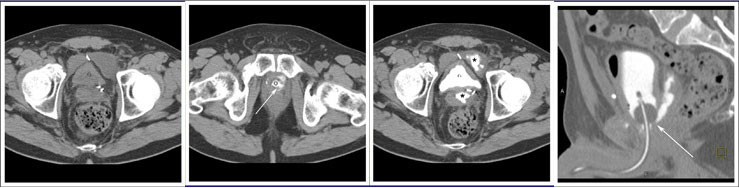 Extraperitoneal anastomotic leak Figure 3. Status post robot-assisted laparoscopic radical prostatectomy (pT2N0Mx, Gleason 6 adenocarcinoma). The patient had abdominal pain after Foley catheter removal, and outside CT showed pelvic fluid collections. CT cystography performed 20 days after surgery. (a) Noncontrast axial CT of the pelvis shows fluid collections anterior and posterior to the bladder. (b) Axial CT csytogram at the level of the vesicourethral anastomosis shows leak from the left posterior aspect of the anastomosis (arrow). (c) Axial CT csytogram above the level of the vesicourethral anastomosis shows contrast accumulation within pelvic fluid collections anterior and posterior to the bladder (asterisk). (d) Lateral volume rendered CT cystogram shows contrast leak from the posterior aspect of the anastomosis, accumulating in the posterior pelvic collection. |
CT CYSTOGRPHIC FINDINGS OF ANASTOMOTIC LEAK Intraperitoneal anastomotic leak
|
CT CYSTOGRPHIC FINDINGS OF ANASTOMOTIC LEAK 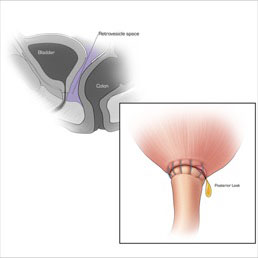 Intraperitoneal anastomotic leak Illustration depicting posterior leak at vesicourethral anastomosis. Sagittal section shows comunication between the retrovesicle space and peritoneal space. |
CT CYSTOGRPHIC FINDINGS OF ANASTOMOTIC LEAK 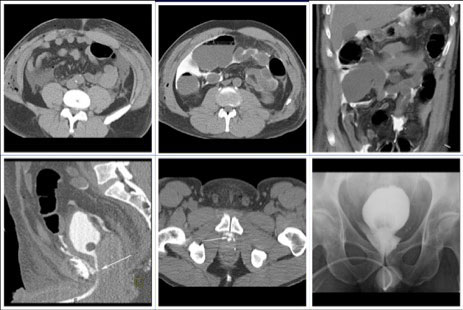 Intraperitoneal anastomotic leak Figure 4. Status post robot-assisted laparoscopic radical prostatectomy (pT2N0Mx, Gleason 6 adenocarcinoma). Urine leak was clinically suspected because of rising creatinine and increase in drain via Davol drain. CT cystography performed 2 days after surgery. (a) Axial noncontrast CT shows moderate ascites. (b) Axial and (c) anterior volume rendered CT cystograms show intraperitoneal leak. (d) Lateral volume rendered and (e) axial CT cystograms show contrast leak from the anterior aspect of the anastomosis (arrow), extending into the peritoneal space indicating intraperitoneal leak. The patient’s condition was improved with conservative management, and the patient was discharged. (f) Follow-up fluoroscopic cystogram performed 18 days after surgery demonstrates decrease in size of leak confined to the extraperitoneal pelvis. No intraperitoneal leak is detected. |
CT CYSTOGRPHIC FINDINGS OF ANASTOMOTIC LEAK 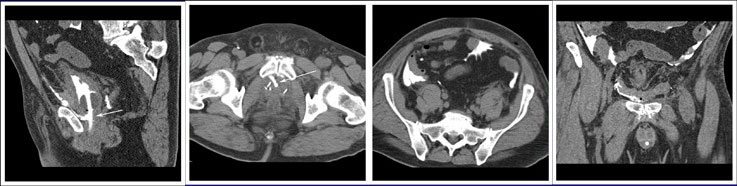 Intraperitoneal anastomotic leak Figure 5. Status post robot-assisted laparoscopic radical prostatectomy (pT2N0Mx, Gleason 6 adenocarcinoma). Urine leak was clinically suspected because of rising creatinine, high output from the drain and low output from a Foley catheter. CT cystography performed 2 days after surgery. (a) Sagittal MPR and (b) axial CT cystograms show leak from the anterior aspect of the anastomosis (arrow) extending to the peritoneal space. (c) Axial and (d) anterior MPR CT cystograms show contrast material within the peritoneal space indicating intraperitoneal leak. The patient’s condition was improved with conservative management, and the patient was discharged. |
CT CYSTOGRPHIC FINDINGS OF ANASTOMOTIC LEAK 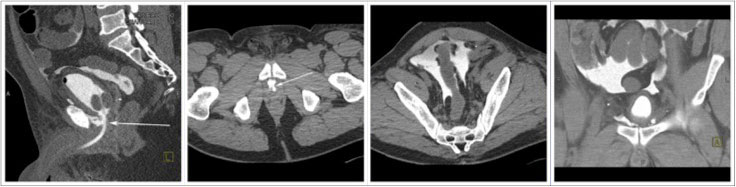 Intraperitoneal anastomotic leak Figure 6. Status post robot-assisted laparoscopic radical prostatectomy (pT2N0Mx, Gleason 6 adenocarcinoma). Urine leak was clinically suspected because of abdominal pain, rising creatinine, high output from a drain and low output from a Foley catheter. CT cystography performed 3 days after surgery. (a) Lateral volume rendered and (b) axial CT cystograms show leak from the anterior aspect of the anastomosis (arrow) extending to the peritoneal space. (c) Axial and (d) anterior volume rendered CT cystograms show contrast material within the peritoneal space indicating intraperitoneal leak. The patient’s condition was improved with conservative management, and the patient was discharged. |
CT CYSTOGRPHIC FINDINGS OF ANASTOMOTIC LEAK 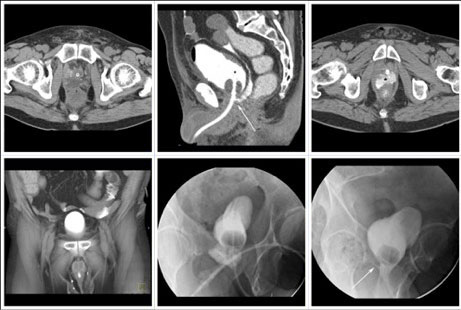 Intraperitoneal anastomotic leak Figure 7. Status post robot-assisted laparoscopic radical prostatectomy (pT2N0Mx, Gleason 8 adenocarcinoma). Urine leak was clinically suspected because of high output from a drain and abdominal pain. CT cystography obtained 6 days after surgery. (a) Noncontrast CT at the level of urethrovesical anastomosis shows small fluid collection posterior to the anastomosis. (b) sagittal MPR and (c) axial CT cystograms show leak from the posterior aspect of the anastomosis (arrow) which accumulates in the pelvic fluid collection (asterisk), and extends to the peritoneal space. (d) Anterior volume rendered CT cystogram shows contrast material within the peritoneal space indicating intraperitoneal leak. The patient’s condition was improved with conservative management, and the patient was discharged. (e) Follow-up fluoroscopic cystography 16 days after surgery and (f) 44 days after surgery shows decrease in size of leak (arrow) confined to the extraperitoneal space. |
CT CYSTOGRPHIC FINDINGS OF ANASTOMOTIC LEAK 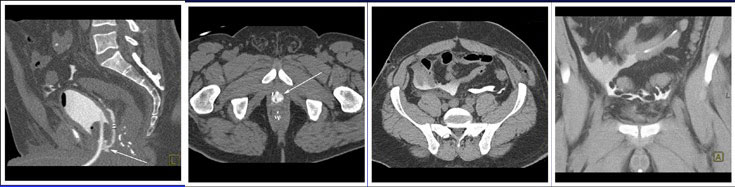 Intraperitoneal anastomotic leak Figure 8. Status post robot-assisted laparoscopic radical prostatectomy (pT2N0Mx, Gleason 7 adenocarcinoma). Urine leak was clinically suspected because of high drain outputs, low Foley outputs, elevated creatinine, and abdominal pain on post op day 1. CT cystography performed 1 day after surgery. (a) Lateral volume rendered and (b) axial CT cystograms show leak from the posterior aspect of the anastomosis (arrow) extending to the peritoneal space. (c) axial and (d) anterior volume rendered CT cystogram show contrast material within the peritoneal space indicating interaperitoneal leak. The patient underwent exploration and reanastomosis. The patient recovered without complication after reanastomosis. |
INCIDENCE AND CLINICAL SIGNIGICANCE OF ANASTOMOTIC LEAK The reported rate of anastomotic leak after open radical prostatectomy is quite varied ranging from 3.9% to 23% in the prior studies [14, 15]. For laparoscopic radical prostatectomy, the reported rate of anastomotic leak occurred in approximately 10% to 17% [10, 16]. Similar complication rates have been reported after robot-assisted laparoscopic radical prostatectomy. |
INCIDENCE AND CLINICAL SIGNIGICANCE OF ANASTOMOTIC LEAK Patil et al. [12] reported 287 (8.6%) of 3327 patients who had robot- assisted laparoscopic prostatectomy had a detectable anastomotic leak on cystography. They also classified anastomotic leaks in patients undergoing robot-assisted radical prostatectomy into three grades based on the gravity cystography [12]. Cystography was obtained 7 days after robotic prostatectomy after instilling 125 to 250 ml of iodinated contrast medium; grade I, extraperitoneal within 6 cm of the urethrovesical anastomosis; grade II, extraperitoneal extending to the side-wall >6 cm from the anastomosis; grade III, intraperitoneal. Among them, 179 (62.4%) was grade I, 84 (29.3%) were grade II, and 24 (8.4%) were grade III. Of the patients with a detectable anastomotic leak, 70% were continent within 3 months and 94% had no involuntary urinary leakage at 1 year. Eight of grade II or II patients (2.8% of 287 patients) in this study required a secondary intervention to correct bladder neck contracture. They concluded that the presence of anastomotic leak might delay the time to continence, but no adverse effect on long-term urinary control, and quantifying the gradation of leakage might provide the prognostic information about patients at risk for future interventions [12]. |
INCIDENCE AND CLINICAL SIGNIGICANCE OF ANASTOMOTIC LEAK
|
INCIDENCE AND CLINICAL SIGNIGICANCE OF ANASTOMOTIC LEAK In our experience, among 882 patients who underwent robot-assisted laparoscopic prostatectomy between 2003 and May 2009, 314 patients (35.6%) underwent postoperative imaging studies to evaluate anastomotic leak including 8 patients who underwent CT cystography. Anastomotic leak was found in 55 patients including 6 patients on CT cystography. This accounted for 55 of 314 patients who had imaging studies (17.5%), and 6.2% of all 882 patients. Leak of contrast material was limited in extraperitoneal pelvic space in 50 patients. Intraperitoneal leak was documented in 5 patients (0.6%) of our patients, and only one of 5 patients required repair of anastomosis. |
Summary Anastomotic leak after robot-assisted laparoscopic prostatectomy is mostly limited to extraperitoneal pelvic space, which is usually transient and requires no further intervention. Rarely, intraperitoneal leak may occur after robot-assisted laparoscopic prostatectomy. Most patients with intraperitoneal leak were treated conservatively. MDCT cystography is a fast and accurate method to detect anastomotic leak and to evaluate extent of extraperitoneal and intraperitoneal leak after RALRP. |
References 1. Su LM, Smithk JA. Laparoscopic and Robotic Radical Prostatectomy and Pelvic Lymphadenectomy. In: Wein AJ, ed. Campbell-Walsh urology. Philadelphia, Pa.: Saunders Elsevier, 2007 2. El-Hakim A, Tewari A. Robotic prostatectomy - a review. MedGenMed 2004;6:20. 3. Abbou CC, Hoznek A, Salomon L, et al. Laparoscopic radical prostatectomy with a remote controlled robot. J Urol 2001;165:1964-1966. 4. Guillonneau B, Vallancien G. Laparoscopic radical prostatectomy: the Montsouris technique. J Urol 2000;163:1643-1649. 5. Colombo JR, Jr., Santos B, Hafron J, Gianduzzo T, Haber GP, Kaouk JH. Robotic assisted radical prostatectomy: surgical techniques and outcomes. Int Braz J Urol 2007;33:803-809. 6. Bhayani SB, Pavlovich CP, Hsu TS, Sullivan W, Su LM. Prospective comparison of short-term convalescence: laparoscopic radical prostatectomy versus open radical retropubic prostatectomy. Urology 2003;61:612-616. 7. Menon M, Shrivastava A, Sarle R, Hemal A, Tewari A. Vattikuti Institute Prostatectomy: a single-team experience of 100 cases. J Endourol 2003;17:785-790. 8. Novara G, Ficarra V, D'Elia C, et al. Evaluating urinary continence and preoperative predictors of urinary continence after robot assisted laparoscopic radical prostatectomy. J Urol;184:1028-1033. 9. Smith JA, Jr., Chan RC, Chang SS, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol 2007;178:2385-2389; discussion 2389-2390. 10. Guillonneau B, Rozet F, Cathelineau X, et al. Perioperative complications of laparoscopic radical prostatectomy: the Montsouris 3-year experience. J Urol 2002;167:51-56. 11. Williams TR, Longoria OJ, Asselmeier S, Menon M. Incidence and imaging appearance of urethrovesical anastomotic urinary leaks following da Vinci robotic prostatectomy. Abdom Imaging 2008;33:367-370. 12. Patil N, Krane L, Javed K, Williams T, Bhandari M, Menon M. Evaluating and grading cystographic leakage: correlation with clinical outcomes in patients undergoing robotic prostatectomy. BJU Int 2009;103:1108-1110. 13. Menon M, Hemal AK, Tewari A, Shrivastava A, Bhandari A. The technique of apical dissection of the prostate and urethrovesical anastomosis in robotic radical prostatectomy. BJU Int 2004;93:715-719. 14. Berlin JW, Ramchandani P, Banner MP, Pollack HM, Nodine CF, Wein AJ. Voiding cystourethrography after radical prostatectomy: normal findings and correlation between contrast extravasation and anastomotic strictures. AJR Am J Roentgenol 1994;162:87-91. 15. Gnanapragasam VJ, Baker P, Naisby GP, Chadwick D. Identification and validation of risk factors for vesicourethral leaks following radical retropubic prostatectomy. Int J Urol 2005;12:948-952. 16. Rassweiler J, Seemann O, Schulze M, Teber D, Hatzinger M, Frede T. Laparoscopic versus open radical prostatectomy: a comparative study at a single institution. J Urol 2003;169:1689-1693. 17. Nadu A, Salomon L, Hoznek A, et al. Early removal of the catheter after laparoscopic radical prostatectomy. J Urol 2001;166:1662-1664. 18. Guru KA, Seereiter PJ, Sfakianos JP, Hutson AD, Mohler JL. Is a cystogram necessary after robot-assisted radical prostatectomy? Urol Oncol 2007;25:465-467. Acknowledgements
|
